Is Enthalpy A State Function
This is a new state function. Enthalpy h is a state function because it is defined solely in terms of other state functions.
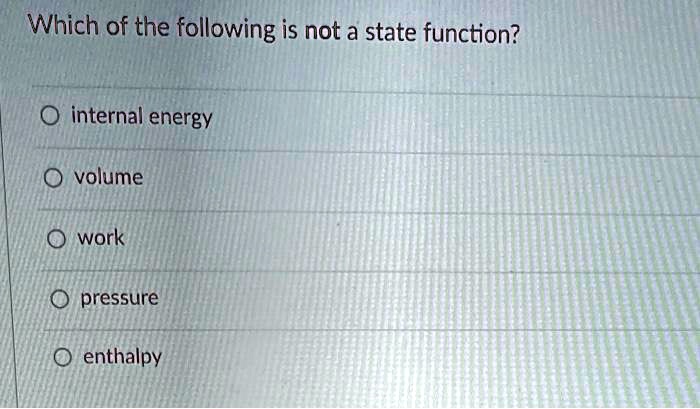
Solved Which Of The Following Is Not A State Function Internal Energy Volume Work Pressure Enthalpy
Enthalpy is a State Function is shared under a not declared license and was authored.

. Using the statefucntion property of enthalpy to calculate enthalpy changes for chemical reactions. Enthalpy as a State Function. This implies that when a system changes from one state to another the change in enthalpy is independent of the path between two states of a.
Enthalpy is a state function. The fact that the internal energy and the enthalpy are both state functions has an important corollary. Enthalpy is a state function because it is defined in terms of state functions.
It means that when a system undergoes any change whatever then the. H U P V. We can define this term as enthalpy.
It is a state function at constant pressure used in chemical and biological systems. As represented by the solution to the integral enthalpy is a state function because it only depends on the initial and final conditions and not on the path taken to. Enthalpy is considered a state function because its current value will only depend upon the final and initial values of heat in a reaction but not the path or process that occurred for it to reach.
The state variable the use of the word function has no function here is called energy of displacement the pressure involved is. The physical state temperature volume and number of particles are the factors that affect entropy. The value of entropy depends only on the initial and final state of.
Enthalpy h is a state function because it is defined solely in terms of other state functions. The answer to this question is yes. Enthalpy H has to do with thermodynamics.
V Where u p and v are the specific internal energy the pressure and the specific. Enthalpy is considered a state function because its current value will only depend upon the final and initial values of heat in a reaction but not the path or process that occurred. As represented by the solution to the integral enthalpy is a state function because it only depends on the initial and final conditions and not on the path taken to.
Difference Between State Function And Path Function. Enthalpy is an energy-like property or state functionit has the. U P and V are all state functions.
The value is noted for initial stage and the final stage. Their values depend only on the state of the system and not. In the thermodynamics of equilibrium a state function function of state or point function for a thermodynamic system is a mathematical function relating several state variables or state.
Density ρ The above is the list of properties that depends on the state. Internal energy u 4. As seen in the above example enthalpy is a state function because its value depends only on initial and final conditions.
In the c See more. Where u p and v are the specific internal energy the pressure and the specific volume. Enthalpy the sum of the internal energy and the product of the pressure and volume of a thermodynamic system.
The state functions are also known as state variables in thermodynamics. This means that enthalpy depends only on.
Chem 245 Enthalpy
Helmholtz And Gibbs Free Energies
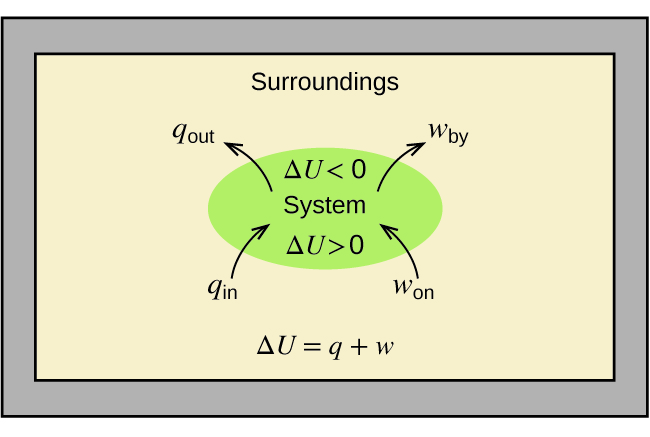
8 3 Enthalpy Chemistry Libretexts
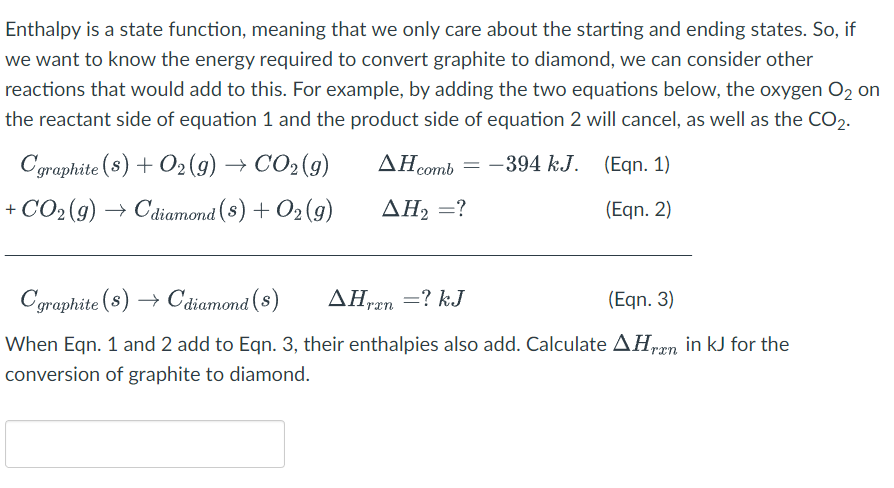
Solved Enthalpy Is A State Function Meaning That We Only Chegg Com
Chem 245 Enthalpy
Chem 245 Entropy

Internal Energy Change In A Cyclic Process Is Zero

Categorize These Properties Into State And Path Functions A Internal Energy B Volume C Heat D Enthalpy E Temperature F Work G Molar Heat Capacity

Solved Question 4 5 Points A4 Choose Which Of The Following Statements Are True About State Functions Enthalpy Is An Example Of A State Function The Rate Of The Reaction Matters All
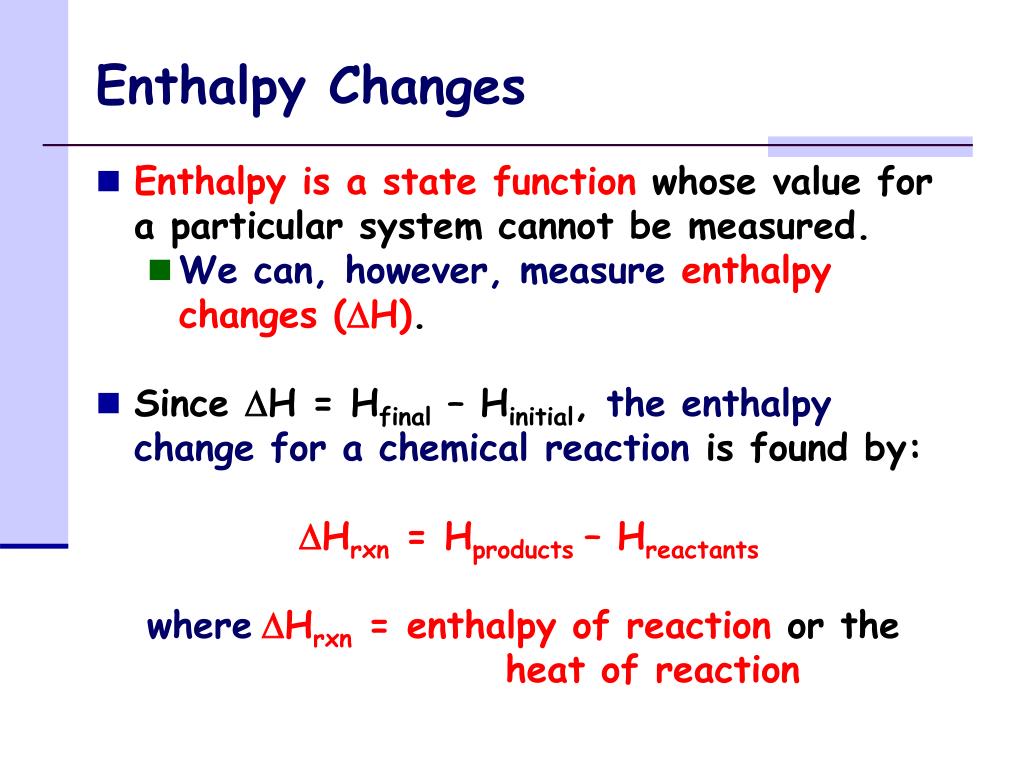
Ppt Enthalpy Changes Powerpoint Presentation Free Download Id 2914427
![]()
Is Enthalpy A State Function Lab 8 Chem 105 Lab Reports Chemistry Docsity
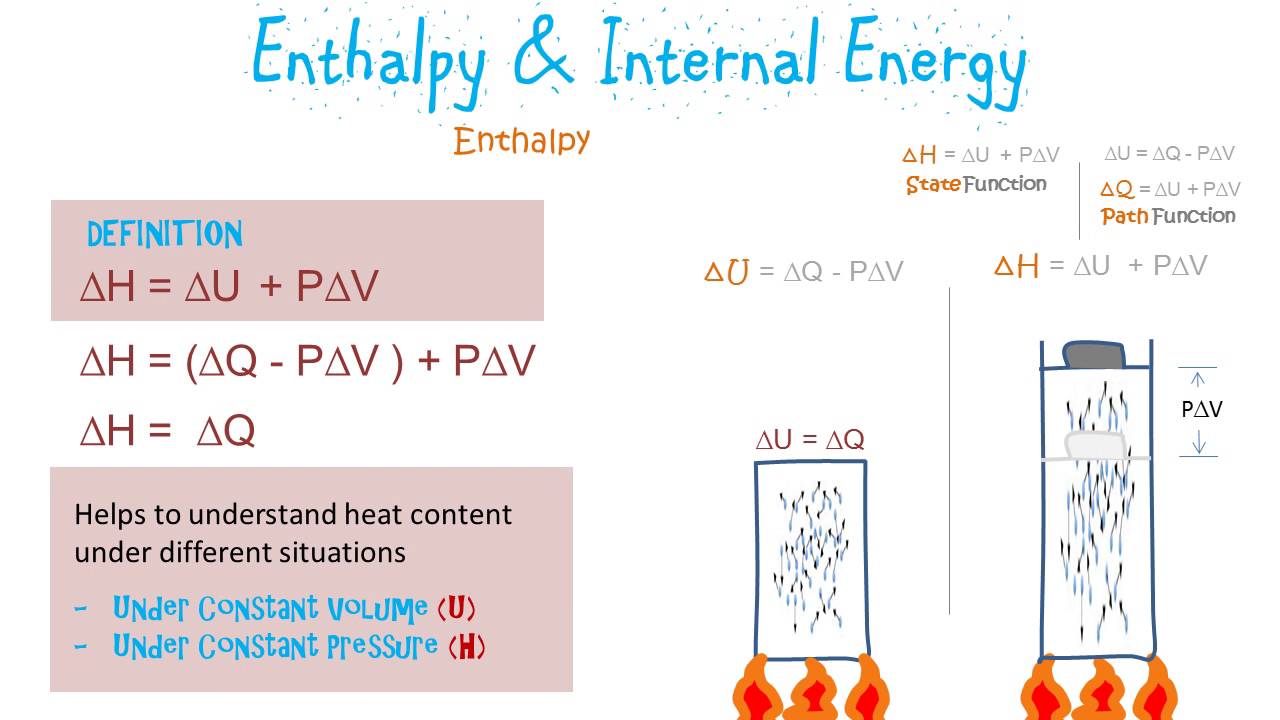
Enthalpy And Internal Energy Youtube

State Versus Path Functions Thermodynamics Discussion And Examples Youtube
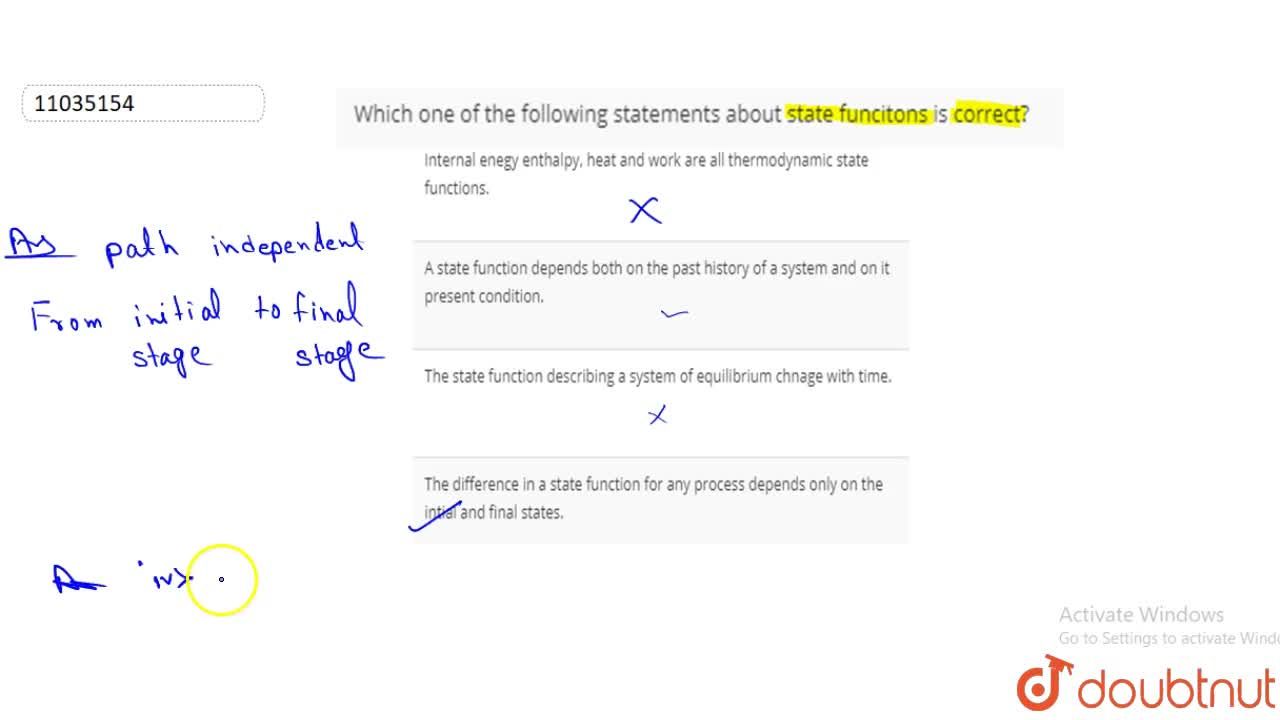
Internal Enegy Enthalpy Heat And Work Are All Thermodynamic State Functions

Which One Of The Following Statements About State Functions Is Correct A Internal Energy Enthalpy Heat And Work Are All Thermodynamic State Functions B A State Function Depends Both On The Past
Explain The State Function Enthalpy H What Is The Relationship Between Deltau And Deltah

Thermodynamics Part 1 Work Heat Internal Energy And Enthalpy